a cornerstone of future treatment.”
McCann said that the next step is to focus on bringing reSET into the hands of patients and clinicians. He hopes Pear Therapeutics will officially launch the product over the next six months.
The FDA’s approval “is really an amazing moment,” he said. “To put it into perspective, this the first time the FDA has approved something like this, especially in addiction treatment.”
Summary
Article Name
FDA approves first-ever mobile app for substance use disorders
Description
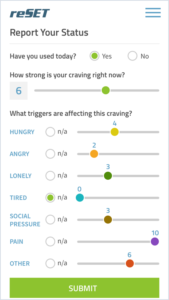
The Food and Drug Administration (FDA) has allowed marketing for the first-ever government-approved mobile medical app designed to help people with a substance use disorder (SUD).
Author
Cesar Gamboa
Publisher Name
Addiction Now