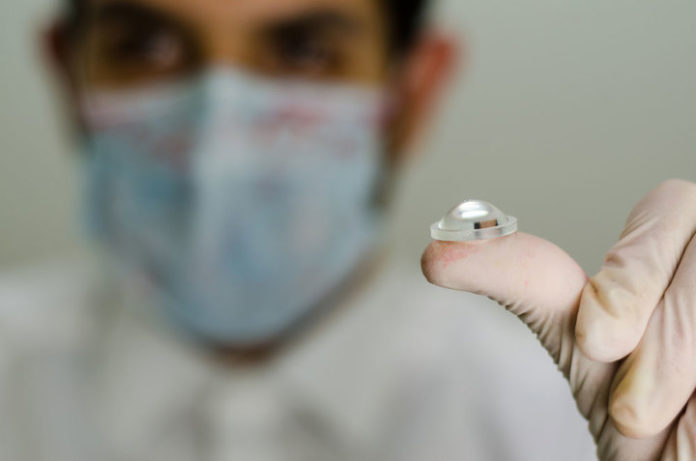
After the Food and Drug Administration (FDA) approved a version of buprenorphine called Probuphine to be used as an implant to treat opioid abuse last May, researchers in Turkey now say they have created a chip implant that can be combined with medication to treat addiction and sustain a long-lasting recovery.
The implants consistently provide a lower dose of the similar drug in order to alleviate the harsher symptoms of going “cold-turkey” while also weaning the patient off opioids strictly as a component of their overall treatment program, which should include intensive therapies and counseling.
After a minor five-minute operation that inserts the chip into the abdominal wall with local anesthesia, the implant administers naltrexone for three months at a specified level while the FDA-approved Probuphine implant requires a much more complex surgery but can treat the patient for twice the amount of time.
Side effects of naltrexone include nausea, headaches, anxiety, and insomnia, while the side effects of the naltrexone implant include pain, itching and redness around the site (upper arm) of the implant as well as depression, constipation, headaches, nausea, vomiting and back pain. The complex surgical procedure can also result in nerve damage.
Opening up availability of medication-assisted (MA) drug addiction treatment like the implant is a key factor of the opioid action plan created by the FDA. The goal is to reduce heroin and prescription opioid painkiller overdoses. Medication-assisted treatment also cuts the risk of death in half according to the Substance Abuse and Mental Health Services Administration (SAMHSA).
The implants not only treat patients with smaller doses of the drug they are dependent upon, it also dulls the pleasurable effects of opioids in order to make them less attractive. The method had been proven to be clinically effective. It also helps improve social functioning, reduces criminal activity, is cost effective, and minimizes risks of spreading diseases like HIV and Hepatitis C — which can be contracted through the blood by sharing needles.
Nonetheless, the National Institute on Drug Abuse (NIDA) reported that 2.2 million Americans over the age of 12 were dependent on opioids in 2014, but fewer than 1 million received medication-assisted treatment. In addition, less than half of private addiction treatment programs have adopted the practice. Within the programs that do offer the recovery method, only 34.4 percent of patients are prescribed medications of any kind.
Despite the generally positive results, SAMHSA reported that medication-assisted treatment dropped from… (continue reading)